Introduction
Viral reactivations, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), adenovirus (ADV), human herpesvirus 6 (HHV6), and BK virus (BKV), constitute significant infectious complications following pediatric hematopoietic stem cell transplant (HSCT). abT cell depletion of haploidentical graft prevents risk of graft-versus-host disease (GVHD) but leads to delayed immune recovery and increased risk of viral reactivations. Donor lymphocyte infusion (DLI) with CD45RA-depleted memory T cells has been reported as an effective method to rapidly recover T cell immunity post HSCT. We hereby report the experience of CD45RA-depleted memory T cell infusion to treat viral reactivation following pediatric abT cell depleted haploidentical HSCT in our center over the last three years.
Methods
We retrospectively reviewed pediatric patients with abT cell depleted haploidentical transplant from related donors in our center from 2021-2023, and those who received memory T cell DLI for one or more viral reactivations. We routinely infuse CD45RA depleted memory T cells (dose at 1x10 6 CD3 T cells/kg) on the day of stem cell infusion prophylactically. Surplus of memory T cells harvested was cryopreserved for future use. Viral reactivations were monitored regularly after transplant by blood PCR assays (twice weekly for CMV; once weekly for EBV, ADV, and HHV-6). BK virus of urine and blood were monitored if patients became symptomatic for hemorrhagic cystitis. Once virus reactivation is identified, we start antiviral medications and infuse cryopreserved donor memory T cells for treatment. If subsequent virus PCR tests remain positive, donor memory T cells are infused every 1-2 weeks until the test is negative.
Results
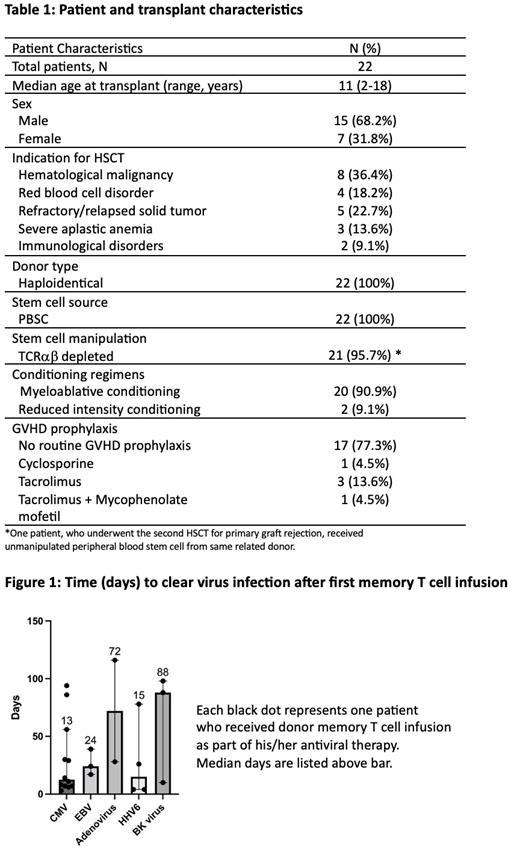
A total of 52 memory T cell infusions were administered to 22 patients for viral reactivation after HSCT. Half of the patients (n=11) required only one infusion for viral clearance, whereas the other half received memory T cell infusion for 2-9 times. 19 patients (86.4%) are alive at time of data collection and analysis (July 2023), and 18 patients (81.8%) are in complete remission. The characteristics of patients and transplants are summarized in Table 1. The viral reactivations, which are intended to treat, include CMV (57.7%), EBV (7.7%), ADV (23.1%), BKV (15.4%) and HHV6 (9.6%), and some patients suffered from multiple viral reactivations at the time of memory T cell infusion (so the total percentage is more than 100%). The median day of memory T cell infusion post-transplant was day 46 (range 4-183). The median and most commonly used cell dose was 2 (0.025 - 20.5) x 10 6/kg CD3+ memory T cells (CD45RA depleted). The median duration to achieve negative PCR test for each virus are 13 days (CMV), 24 days (EBV),72 days (ADV), 15 days (HHV-6) and 88 days (BKV) (Figure 1). Thus most of the patients were CMV PCR negative within 15 days after DLI. All DLI were well tolerated. One patient (4.5%) was reported to suffer from stage 2 skin GVHD post donor memory T cell infusion.
Conclusions
CD45RA-depleted donor memory T cell infusion is a safe, feasible and effective treatment for viral reactivation following pediatric haploidentical stem cell transplant.
Disclosures
Leung:Miltenyi: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal